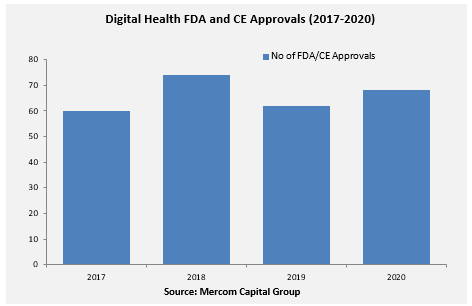
A total of 68 digital health products received FDA/CE approvals in 2020, compared to 63 in 2019, according to Mercom’s Q4 and Annual 2020 Digital Health Funding Report.
Clinical Decision Support companies received the most FDA approvals in 2020 with 21, followed by Mobile Wireless and Medical Imaging with 13 each, Wearable Sensors with 12, and mHealth Apps with six.
Since Q1 2017, over 250 Digital Health products have received FDA/CE approvals. Learn More.
Alphabet’s research organization Verily Life Sciences received FDA 510(k) clearance for its Verily Study Watch. The Watch is designed to record, store, transfer, and display single-channel ECG rhythms. Alphabet, through its VC investment arm, GV (formerly Google Ventures), has invested almost $2 billion in more than 40 digital health companies since 2010.
Abbott, a medical device and healthcare research company, received FDA clearance for its wearable FreeStyle Libre 3 patch, a real-time glucose monitoring system for people with diabetes. To date, a total of seven Abbott’s digital health products have been approved by the FDA. Recent Approvals include – FreeStyle Libre 2 and Abbott Patient Controller App.
Philips, a consumer electronics company, received 510(k) clearance from FDA to market a wide range of its ultrasound solutions (including the Affiniti series, Lumify, CX50, EPIQ series, and Sparq diagnostic ultrasound systems, and off-cart solutions like QLAB Advanced Quantification Software). According to Philips, these solutions will help manage COVID-19-related lung and cardiac complications, according to the company.
According to the Mercom database, Philips has acquired 13 digital health companies since 2010 and has invested over $100 million in 13 digital health startups since 2016.
Fitbit, a consumer electronics and digital fitness tracker maker, received 510(k) clearance from the FDA and CE marking from the European Union for its electrocardiogram (ECG) app. The app assesses heart rhythm for atrial fibrillation, according to the company.
Fitbit, backed by Qualcomm Ventures, SAP Ventures, SoftBank Capital, Foundry Group, and True Ventures, with more than $70 million in funding, went public (in 2015) through IPO, raising $841 million. In 2020, Google acquired Fitbit for $2.1 billion.
Samsung announced that it had received clearance from the FDA for its Samsung Galaxy Watch 3, including a heart-monitoring electrocardiogram (EKG or ECG) app. To date, Samsung has invested over $600 million in more than 20 digital health companies, including Preventice Solutions, Healthy.io, HealthifyMe, Glooko, Cohero Health, and WellDoc.
Ezra, an early cancer screening technology company using MRI, received FDA 510(k) clearance for its Artificial Intelligence (AI). The AI is designed to assist radiologists in analyzing and segmenting prostate MRI. Ezra is backed by$18 million in venture capital from FirstMark Capital, an early-stage venture capital firm.