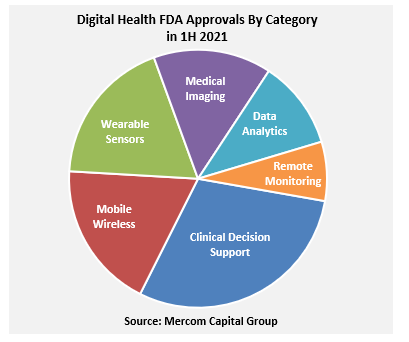
A total of 27 digital health products received FDA approval in the first half (1H) of 2021, compared to 28 in 1H 2020, according to Mercom 1H 2021 Digital Health Funding & M&A Report.
The digital health companies focused on Clinical Decision Support software received 8 FDA approvals during 1H 2021. Mobile Wireless and Wearable Sensors received five FDA approvals each, followed by four Medical Imaging products that received FDA approvals. Three Healthcare Data Analytics software products received FDA approvals, followed by the Patient Remote Monitoring system with two approvals in 1H 2021.
 Notable FDA approvals during 1H 2021
Notable FDA approvals during 1H 2021
Activ Surgical, a developer of surgical intelligence software powered by machine learning, received the FDA 510(k) clearance for its ActivSight Intraoperative Imaging Module. The company’s FDA-approved technology provides surgeons real-time intraoperative visual data and imaging, helping to improve patient outcomes and safety in the operating room. Activ Surgical is backed by nearly $30 million funding from investors, including ARTIS Ventures, LRVHealth, DNS Capital, GreatPoint Ventures, and Tao Capital Partners.
Ava, a women’s reproductive health technology startup, received FDA clearance for its Ava Fertility Tracker, a machine learning-powered fertility tracking sensor bracelet and accompanying app. According to the company, the bracelet is designed to help women in ovulation prediction and conception facilitation. The bracelet, worn only during sleep, provides women with real-time, personalized information about fertility, pregnancy, and general health – delivered in a way that is convenient and non-invasive. Ava is backed by over $30 million funding from investors including btov, SVC, DCM Ventures, Khosla Ventures, and Innovation Endeavors.
RadNet’s subsidiary DeepHealth, a developer of artificial intelligence for mammography interpretation, received FDA clearance for its mammography triage software Saige-Q. Saige-Q is a screening worklist prioritization tool that enables radiologists to manage their mammography cases more effectively using artificial intelligence.
Ultromics, an AI-enabled cardiovascular imaging solutions provider, announced that its EchoGo Pro, an AI-powered decision support tool for clinicians, has been approved by the FDA and is available to clinicians across the U.S. according to the company, EchoGo Pro was trialed and validated in the U.K. and U.S., improving the diagnostic accuracy of clinicians for coronary artery disease, the most common form of heart disease and a leading cause of death in the U.S.
Theranica, which is developing electroceuticals for migraine and other pain conditions, received the FDA clearance for its flagship product Nerivio. Nerivio is a smartphone-controlled prescription wearable device for acute migraine treatment of episodic or chronic migraine in people 12 years and older. Theranica is backed by over $35 million from aMoon Fund, Lightspeed Venture Partners, LionBird, Corundum Open Innovation, Takoa, and other investors.
Since Q1 2017, a total of 291 digital health products have received FDA/CE approvals. Click here to learn more.
Image Credit: Monogram Health