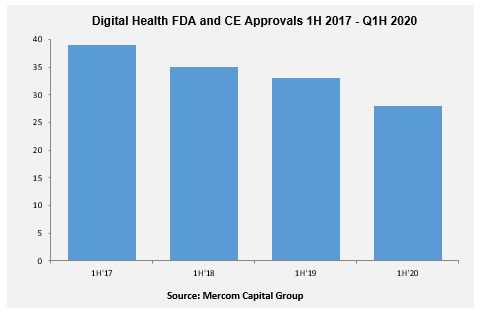
Twenty-eight digital health products received FDA/CE approvals in 1H 2020, compared to thirty-two in 1H 2019, according to Mercom’s Digital Health Q2 and 1H 2020 Report.
Of the 28 products received FDA/CE approvals during 1H, nine were powered by artificial intelligence (AI).
Wearable device makers and medical imaging companies received the most FDA approvals in 1H 2019 with seven each, followed by clinical decision support tool and mobile wireless with five each and mHealth apps with four.
 Notable FDA Approvals In 1H 2020
Notable FDA Approvals In 1H 2020
Verily Life Sciences, an Alphabet’s research organization dedicated to studying life sciences, received FDA 510(k) clearance for its Verily Study Watch. According to Verily, the Study Watch is a prescription-only device intended to record, store, transfer, and display single-channel ECG rhythms, and is designed for only healthcare professionals, adult patients with known or suspected heart conditions.
Alphabet, through its venture capital investment arm, GV (formerly Google Ventures), has invested almost $2 billion in over 40 digital health companies since 2010.
Philips, a Dutch consumer electronics company, received 510(k) clearance from FDA to market a wide range of its ultrasound solutions, including the EPIQ series, Affiniti series, Lumify, CX50, and Sparq diagnostic ultrasound systems, and off-cart solutions like QLAB Advanced Quantification Software. These ultrasound solutions are intended to manage COVID-19-related lung and cardiac complications. According to Mercom database, Philips has acquired 13 digital health companies since 2010. Philips has also invested over $100 million in 13 digital health startups since 2016.
Welldoc, a chronic care management app, announced that FDA cleared an additional feature for its diabetes management app BlueStar Rx, a non-prescription version of BlueStar. WellDoc has raised a total of over $60 million funding to date from Johnson & Johnson Innovation, Samsung Ventures, Merck Global Health Innovation (GHI) Fund, and others.
The FDA has granted 501(k) clearance to Aidoc’s AI-based system for the detection of large-vessel occlusion (LVO), ischemia strokes that result from a blockage in one of the major arteries of the brain. The Israeli startup Aidoc, which provides AI-powered medical imaging solutions for radiologists, has raised $40 million since its founding in 2016.
Propeller Health (formerly Asthmapolis) – a developer of chronic obstructive pulmonary disease and asthma monitoring sensors – received 510(k) clearance from FDA for a sensor and app intended for use with AstraZeneca’s Symbicort inhaler for asthma and COPD. Propeller Health, which had raised over $60 million VC funding, was acquired by ResMed in late 2018, for $225 million.
Since Q1 2017, a total of 227 digital health products received FDA/CE approvals. Learn More