The U.S. Food and Drug Administration (FDA) has approved almost 200 digital health products that include everything from wearable sensors to artificial intelligence-enabled medical imaging technologies since 2017.
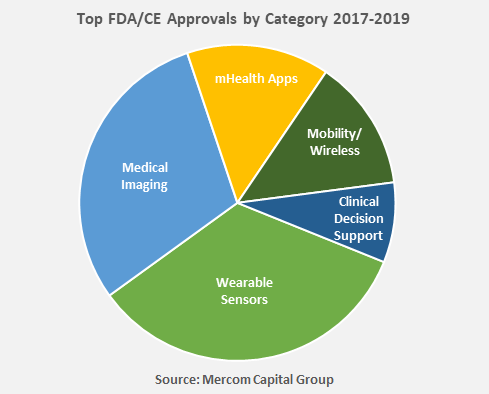
Wearable Sensor enabled technologies have received the most approvals with 58, followed by Medical Imaging technologies with 51. Twenty-five mHealth Apps have also received approvals.
Other digital health categories that received most approvals included connected wireless devices, and clinical decision support software.
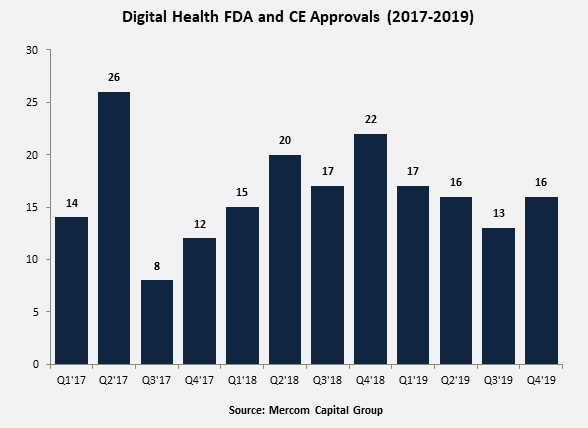
In 2019, 63 digital health products received FDA approvals, compared to 74 in 2018. In 2017, a total of 59 products received approvals.
Notable FDA clearances:
Since 2017, Wearables technologies such as smartwatch and wrist bands have received close to 60 approvals and also raised $1.4 billion in VC funding. Top tech brands such as Apple, received approval for its Apple Watch Series 4, a smartwatch that can perform ECGs, detect atrial fibrillation, and contact emergency services in the case of a fall.
Alphabet’s Verily received 510(k) clearance for its Study smartwatch. The smartwatch helps healthcare professionals as well as adults with known or suspected heart conditions. Alphabet, through its investment arm GV (formerly Google Ventures), has invested $1.4 billion in 37 digital health companies according to Mercom funding database.
Apart from Wearables, Medical Imaging technologies providing X-rays, scanning, image processing solutions received several clearances. Philips, which acquired 16 digital health companies since 2016, received 510(k) approval for its medical imaging products IntelliSpace Portal 9.0 and a range of innovative applications for Radiology in the U.S.
Pharmaceutical company Sanofi received FDA clearance for the insulin dose calculator app – My Dose Coach.
Medical device company DexCom which acquired TypeZero Technologies in 2018 for an undisclosed amount, announced FDA clearance for its mobile app Dexcom G5. The app allows diabetes patients to monitor and manage their glucose levels on their mobile devices. The company also received approval for Dexcom G6 Pro, a wireless glucose monitoring system for diabetes patients.
Siemens Healthineers, which acquired four digital health companies, including Epocal, and Medicalis also received FDA clearance for its AI-Rad Companion Chest CT, an intelligent software assistant from that brings artificial intelligence to CT.
Recently U.S. Food and Drug Administration released a set of guidance to continue to foster innovative approaches to the development of digital health tools.