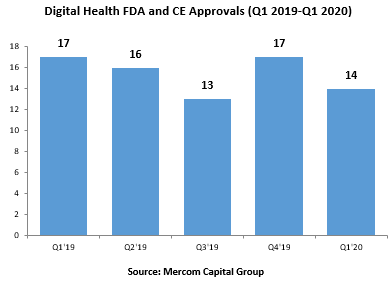
A total of 14 digital health products received FDA approval in Q1 2020, compared to 17 in Q4 2019, according to Mercom Q1 Digital Health Funding & M&A Report.
Since Q1 2017, over 200 digital health products have received FDA/CE approvals. Click here to learn more.
The digital health companies focused on the Clinical Decision Support Software received the most FDA approvals during Q1, followed by Wearable Sensor Devices.
Mobile Wireless Devices (monitors the patient’s health and sends information to a companion app) and Medical Imaging software, received two FDA approvals each.
One mHealth app also received FDA approval in Q1.
Notable FDA approvals during Q1 2020
Verily Life Sciences, also known as Verily, an Alphabet’s research organization devoted to the study of life sciences, announced that the Verily Study Watch had received 510(k) clearance from the FDA as a Class II medical device for its on-demand ECG feature. Specifically, the FDA-cleared Study Watch is a prescription-only device intended to record, store, transfer, and display single-channel ECG rhythms. The watch is designed for healthcare professionals and adult patients with known or suspected heart conditions and health-conscious individuals.
The digital therapeutics startup, Pear Therapeutics, received FDA approval for its Somryst, the prescription digital therapeutic (PDT) app intended for aged patients who have chronic insomnia.
Eko Health, a digital health company, applying artificial intelligence (AI) in the fight against heart disease, announced that the FDA had cleared a suite of algorithms. When combined with Eko’s digital stethoscopes, the algorithms will enable healthcare providers in the U.S. to more accurately screen for heart conditions during routine physical exams.
Aidoc, a provider of AI-enabled medical imaging solutions for radiologists, received FDA clearance for its AI solution for flagging Large-Vessel Occlusion (LVO) in head CTA scans. Combined with Aidoc’s previously-cleared AI module for flagging and prioritizing intracranial hemorrhage, they provide a comprehensive AI package for the identification and triage of both ischemic and hemorrhagic stroke in CTs, speeding time to treatment when every minute counts.
Respiratory monitor maker NuvoAir (formerly Pond Healthcare Innovation) secured FDA 510(k) clearance for its Air Next, a Bluetooth-enabled digital respiratory health tracking device designed to predict worsening respiratory conditions; enabling medical professionals to focus on their most critical or severe cases.